- Home
- About
- Partners
- Newcastle University
- University of L'Aquila
- University of Manchester
- Alacris Teranostics GmbH
- University of Pavia
- Polygene
- Consiglio Nazionale delle Ricerche
- INSERM
- Certus Technology
- Charité Universitaet Medizin
- GATC Biotech
- University Medical Center Hamburg Eppendorf
- Evercyte GmbH
- University Hospital of Cologne
- PRIMM Srl
- University of Freiburg
- University of Antwerp
- Finovatis
- Research
- SYBIL at a glance
- Bone
- Growth plate
- Desbuquois dysplasia
- Diastrophic dysplasia
- MCDS
- Osteopetrosis
- Osteoporosis
- Osteogenesis imperfecta
- Prolidase deficiency
- PSACH and MED
- Systems biology
- SOPs
- Alcian Blue staining
- Bone measurements
- BrdU labelling
- Cell counting using ImageJ
- Chondrocyte extraction
- Cre genotyping protocol
- DMMB assay for sulphated proteoglycans
- Densitometry using ImageJ
- Double immunofluorescence
- Electron microscopy of cartilage - sample prep
- Extracting DNA for genotyping
- Grip strength measurement
- Histomorphometry on unon-decalcified bone samples
- Immunocytochemistry
- Immunofluorescence
- Immunohistochemistry
- Quantitative X-ray imaging on bones using Faxitron and ImageJ
- Skeletal preps
- TUNEL assay (Dead End Fluorimetric Kit, Promega)
- Toluidine Blue staining
- Toluidine Blue staining
- Von Kossa Gieson staining
- Wax embedding of cartilage tissue
- Contact Us
- News & Events
- Links
- Portal
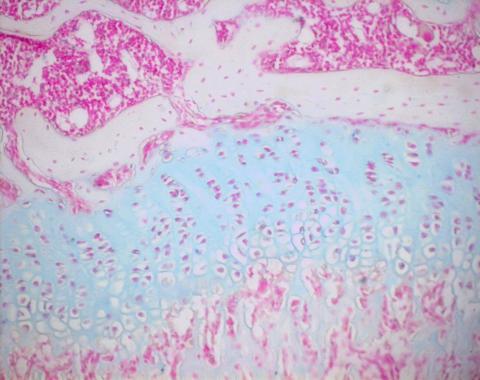
Alcian Blue staining
Sample preparation
- sacrifice the animals and dissect knee samples
- fix in 4%PFA over 48h in the fridge
- decalcify in 20% EDTApH7.4 for 2 weeks (shaking)
- wax embed and section (6um sections)
Note
to generate reliable data, this experiment should be performed on matched sections from 3 unrelated animals per genotype
Alcian Blue
15mg Alcian Blue
80ml 95% EtOH
20ml glacial acetic acid
adjust pH to 0.75
Note
pH 0.75 ensures Alcian Blue specificity to sulphated proteoglycans and is a recommended pH for staining cartilage. At pH 2.5 it stains most acid mucins (except some of the strongly sulphated groups), at pH 1.0 it will stain only weakly and strongly sulphated acid mucins, and at pH 0.2 it will only stain strongly sulphated acid mucins.
Staining steps
dewax in xylene 2 x 5min
. ↓
100% EtOH 3min
. ↓
90% EtOH 3min
. ↓
70% EtOH 3min
. ↓
50% EtOH 3min
. ↓
dH2O 2x 3min (from here on, do not let the samples dry!)
. ↓
Alcian Blue 10min
. ↓
dH2O 2x 5min
. ↓
Fast Red 5min
. ↓
dH2O 2x 5min
. ↓
95% EtOH 3min
. ↓
100% EtOH 5min
. ↓
dehydrate in xylene 2x 5min
. ↓
coverslip, dry overnight
End result: nuclei red, sulphated proteoglycans blue